Shaping a world free from fear of infection
-Delivering rapid and accurate diagnosis to all those fighting against illness-

Message from CEO & Founder
Founded in 2016, Nanotis has been conducting research and development of next-generation biosensing technology collaborating with the University of Tokyo. As its first application, we are advancing the development of Point-of-Care testing devices for respiratory infections, including influenza viruses and Covid-19.
Due to Covid-19, the importance of accurate, rapid, simple, and cost-effective testing has been globally recognized. We aim to offer genuinely valuable technology to serve patients, those concerned about infection, frontline healthcare professionals, and government bodies involved in infection control efforts. Our ultimate goal is to play a pivotal role in early infection detection and prevention, making a substantial contribution to public health.
I studied physics in graduate school and had experience in management at financial institutions and startup. Influenced by family members who are physician and engineer, I developed a strong desire to create products that can help people in need around the world. With this passion, I made the decision to found Nanotis. Currently, based in the laboratory at Faculty of Engineering in the University of Tokyo, our team is dedicated to product development, aiming to provide innovative testing solutions to the world.
Our pursuit is for the realization of rapid and accurate testing. Nanotis is dedicated to the ongoing challenge of creating a society where people can live without the fear of infectious diseases.
Nanotis Corporation CEO & Founder Lisa Sakashita
Core technology targeting a wide range of viruses:NANOTIS method
We possess the world’s first infectious disease digital testing technology, called “Nucleic Acid Navigated Optically Traceable Immuno-Sensing (abbreviated as NANOTIS method)
We introduced a concept of physical concentration in a specific detection system for the first time in the world, and achieved the concentration of virus nucleoprotein, a molecule on the scale of several nanometer, which was previously considered technically impossible (patent submitted). Also, it is important to highlight the remarkable versatility of this technology, as it can target not only viruses but also bacteria, biomarkers, and among others.
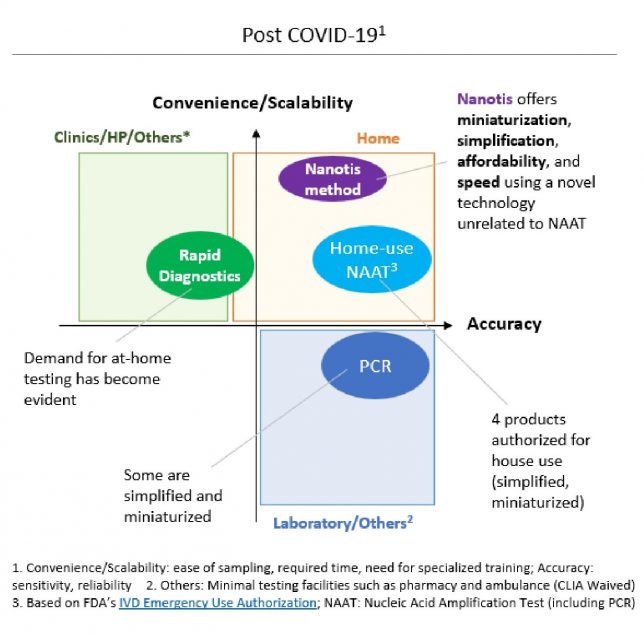
In the field of infectious disease testing, existing technologies such as genetic amplification (PCR, etc.) have faced challenges of time, cost, and the need for specialized expertise. Antigen tests (immunochromatography) have lacked sufficient sensitivity and scalability.
Nanotis method adopts a theoretical approach similar to genetic amplification in terms of increasing the overall sensitivity. However, the technology is fundamentally different, ensuring the advantages of simplicity and cost-effectiveness similar to antigen tests while providing unique advantages of high sensitivity, ultra-rapid results, and efficient data integration.
During the COVID-19 pandemic, a few home-use products were authorized. While these products offer simplicity and accuracy, they encounter challenges related to cost, time, and difficulty of sample collection. In this regard, our approach can deliver unique value to users of such tests.

Nanotis provides scalable screening tests to a wide range of entities, including healthcare institutions, individuals, businesses, care facilities, educational institutions, event venues, and more, ushering in a new era of value. Additionally, as our technology can send digital epidemiological data instantly and automatically, it serves as the core for the next-generation infrastructure of infectious disease surveillance in public administration, addressing the challenges related to speed and accuracy that existing technologies have faced and laying the foundation for a novel testing ecosystem.

We have a technology portfolio that extends the foundation of the NANOTIS method to detect a wide range of biological particles, including bacteria, biomarkers, and nucleic acids. We are strategically filing patents for this expansion.